Which Statement Best Describes an Oxidation Reduction Reaction
A Electrochemistry is the study of only oxidation reactions. Chemistry 19092021 2310 NetherisIsTheQueen.
A chemical reaction in which there are fewer products than reactants B.

. Which of the following statements best describes how a reducing agent in is chemically altered in a biological redox reaction. So reducing agent is a substance that produces others and oxidize itself. When an element is oxidized it loses electrons.
If there is formation of a precipitate the reaction is an oxidation-reduction reaction. 1Which statement best describes an oxidation-reduction reaction. However an oxidizing agent oxidizes something else and gets reduced therefore gaining electrons.
Cl2 2e 2Cl C. Which statement best describes what happens during the redox reaction between nadh and complex i of the electron transport chain. A chemical reaction that involves oxygen C.
A chemical reaction in which electrons are released from the system D. Which statement best describes what changes occur over the course of the following oxidation-reduction reaction. Mg Mg2 2e D.
So here first we will discuss that What is reducing agent. This is the basis of redox reactions. 6 What type of bond is formed when electrons are transferred from one atom to another explain.
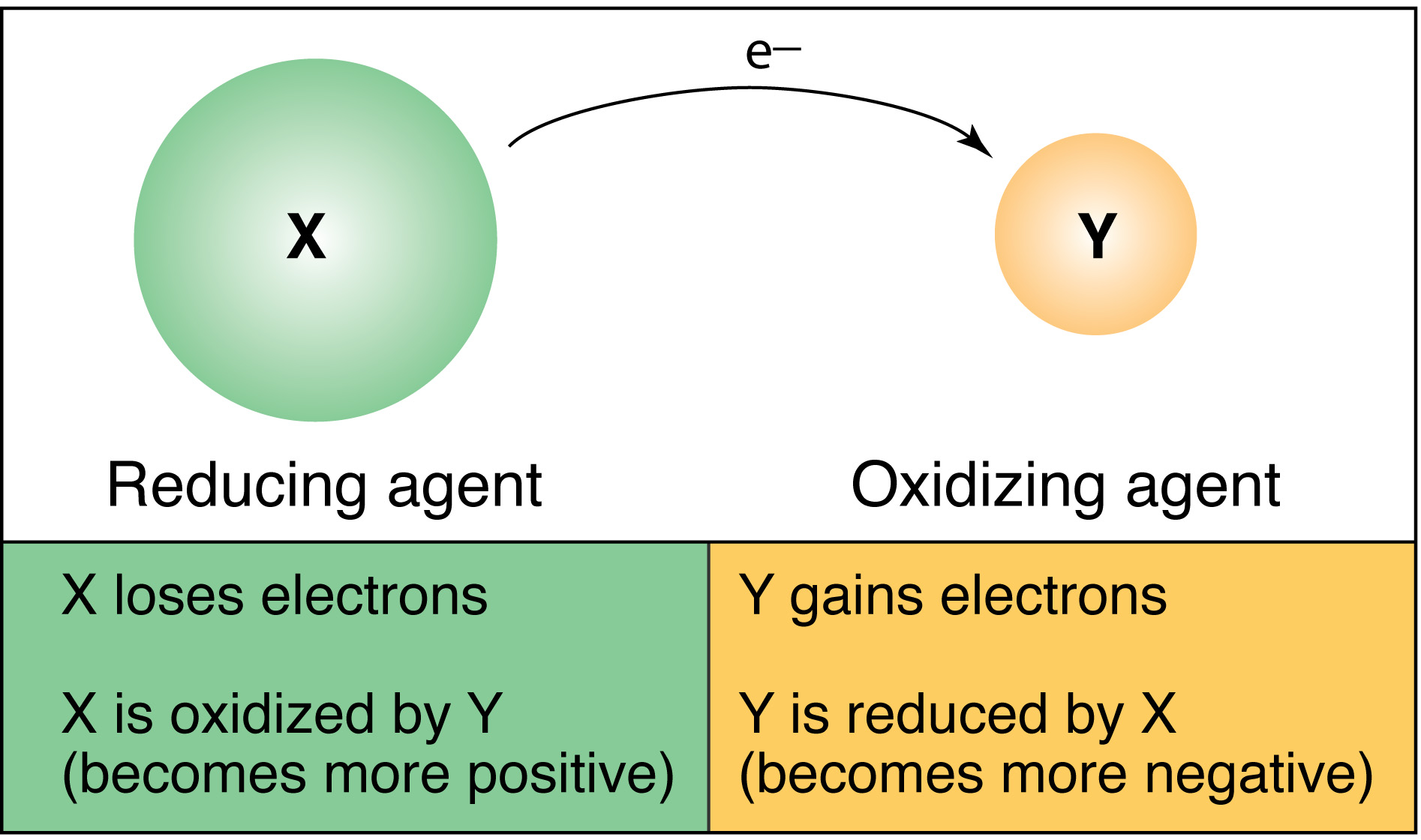
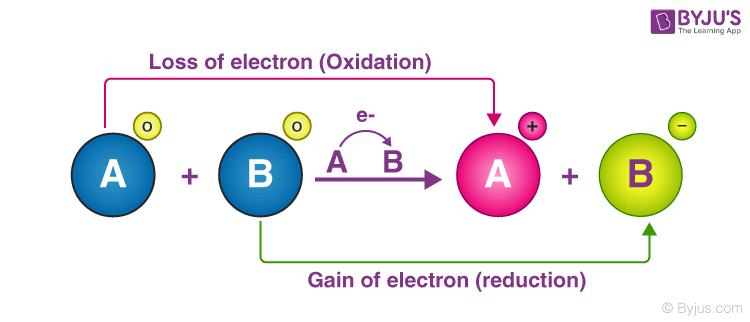
Silver ion Ag is a stronger oxidizing agent than copper ion Cu2 and copper metal is a stronger reducing agent than silver. Third option is the correct one. An oxidation-reduction redox reaction is a type of chemical reaction in which electrons are transferred between chemical species.
In this reaction iron is reduced and copper is oxidized In this reaction both iron and copper are oxidized Which statement best describes the following reaction. Which of the following is true about a redox reaction. This is a redox reaction in which octane C8H18 is oxidized.
The sharing of a pair of electrons between two atoms a relatively weak bond. Which statement best describes the reaction represented by the equation2NaCl 2H2O electricity -- Cl2 H2 2NaOH. C Iron transfers two electrons to copper.
A Electrochemistry is the study of only oxidation reactions. 8 What happens after the transfer of electrons. 1Which statement best describes an oxidation-reduction reaction.
These reactions involving electron transfers are known as oxidation-reduction or redox reactions. It permits the migration of ions In what kind of cell are the redox reactions made to occur by an externally applied electrical current. A chemical reaction that involves oxygen C.
In the reaction MgCl2MgCl2 the correct half-reaction for the oxidation that occurs is A. 9 What happens when electrons are transferred in a chemical reaction. Behave given christian S here which of the following statements best describes how our reducing agent in is chemically altered in a biologically dogs reaction.
Nadh is oxidized as a pair of electrons which are transferred to the etc and 4 four h are pumped into the intermembrane space. O O O If there is a change in the oxidation states of atorns from reactants to products the reaction is an oxidation- reduction reaction If there is formation of salt and water the reaction is an oxidation-reduction reaction. Which statement best describes the relationship between electrochemistry and oxidation-reduction reactions.
The sharing of a pair of electrons between two atoms a relatively strong bond. B Electrochemistry is the study of only reduction reactions. Chemistry 25022021 1840 olivia0420.
7 What happens to the electrons in a covalent bond. The type of reaction that is shown is. When an object is electroplated the occurrence of a redox reaction is nonspontaneous and it requires an electric current.
C The reaction occurs in an electrolytic cell and releases energy. In a redox reaction an electron is lost by the reducing agent. A chemical reaction in which electrons are released from the system.
In redox reactions a reduced half and an oxidized half occur together. Which of the following statements best describes an ionic bond. Olt gains a hydrogen atom and loses potential energy O It loses a hydrogen atom and loses potential energy It loses a hydrogen atom and gains potential energy.
C decomposition reaction. A chemical reaction in which electrons are transferred between reactants 2Beryllium Be has four electrons. D Copper transfers two electrons to iron.
A The reaction occurs in a voltaic cell and releases energy. The presence of which reactant is the best indicator of an oxidation-reduction reaction. But when an element is reduced it gains electrons.
Which answer best describes what is happening in the following reaction. The correct statement that describes a Redox reaction is D. A chemical reaction that involves oxygen.
A Redox reaction oxidation-reduction reaction involves the exchangetransfer. The chemical formula that shows the correct subscripts is D BeF₂. B The reaction occurs in a voltaic cell and absorbs energy.
4Al 302 2Al2O3 In this reaction aluminum is oxidized and oxygen is reduced In this reaction aluminum is reduced and oxygen is oxidized In this reaction th aluminum and oxygen are oxidized This is not an. Which statement best describes how a salt bridge maintains electrical neutrality in the half cells of an electrochemical cell. The reaction that takes place in a chemical cell is best classi ed as A.
Which statement best describes the oxidizing and reducing abilities of the reactants. Fe Cu2 Fe2 Cu A Two electrons are lost. Nadh is oxidized by fadh2 which is then immediately oxidized in complex complex ii.
The attraction between two charged atoms a relatively weak bond in an aqueous solution. B Electrochemistry is the study of only reduction reactions. A chemical reaction in which electrons are released from the system D.
Because of this in many cases H 2 O or a fragment of an H 2 O molecule H or OH in particular can participate in the redox reactionAs such we need to learn how to incorporate the solvent into a balanced redox equation. O lt gains a hydrogen atd and gains potential energy. A chemical reaction in which there are fewer products than reactants B.
Which equation represents the half-reaction that takes place at. B Iron changes into copper. A chemical reaction in which electrons are transferred between reactants.
Which statement best describes the relationship between electrochemistry and oxidation-reduction reactions. 5 What statement best describes the actions of electrons in an ionic bond. So here reducing agent is a substance a substance that reduces other and oxidized itself.
E Two electrons are gained. A chemical reaction in which electrons are transferred between reactants. 1Which statement best describes an oxidation-reduction reaction.
A chemical reaction in which there are fewer products than reactants. C Oxidation-reduction reactions are the basis of electrochemical cells.

Chemistry Reduction And Oxidation Reactions Wikiversity

1 Which Statement Best Describes An Oxidation Reduction Reaction 1 Point A A Chemical Reaction In Brainly Com
Comments
Post a Comment